|
The liver is the largest organ in the body and performs a variety of tasks that impact all parts of the body. As a result, liver disease has widespread effects on virtually all other organ systems. Remarkably, the liver is the only organ that can regenerate itself. Numerous attempts have been made over the past 30 years to develop a surrogate technique to provide either partial or total metabolic support for the patient with a failing liver. The technical and clinical objective is to provide a temporary liver assist until a patient's own liver regenerates or until a liver transplant can be performed.
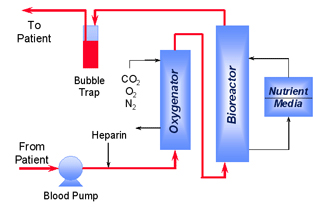 The Excorp Medical Bioartificial Liver System comprises an extracorporeal (outside the body) process for continuously withdrawing a patient's whole venous blood, maintaining temperature, oxygenating to arterial levels, adjusting pH and perfusing a hollow fiber bioreactor charged with primary porcine hepatocytes before returning the blood to the patient. Porcine hepatocytes are obtained from qualified animals produced in a high health status herd. The Excorp Medical Bioartificial Liver System comprises an extracorporeal (outside the body) process for continuously withdrawing a patient's whole venous blood, maintaining temperature, oxygenating to arterial levels, adjusting pH and perfusing a hollow fiber bioreactor charged with primary porcine hepatocytes before returning the blood to the patient. Porcine hepatocytes are obtained from qualified animals produced in a high health status herd.
Approximately 100 grams of hepatocytes are infused into the hollow fiber cartridge. Viability, oxygen consumption and other parameters are monitored to establish potency of each hepatocyte preparation and the resulting Bioreactor. The hollow fiber membrane serves as an immunoisolation barrier between the two species. High blood flows are maintained during clinical hemoperfusion with a therapeutic procedure designed to last for 12 hours. The instrument console provides control of the process, detecting potentially hazardous conditions and alerting the operator to appropriate corrective actions.
The technology is protected by issued US and European patents. The patent describes a platform technology of high-density cell culture that can be extended beyond liver cells to a wide variety of other cell types including pancreatic islets and other endocrine cells. The Company's bioartificial liver system has also been designated an "Orphan Product" by the FDA for the treatment of acute liver failure. Among the several benefits of the Orphan Drug Act, is a seven year market exclusivity following FDA marketing approval.
|





